PMMA-based composite bone cements
Title: PMMA-based composite bone cements
Description:
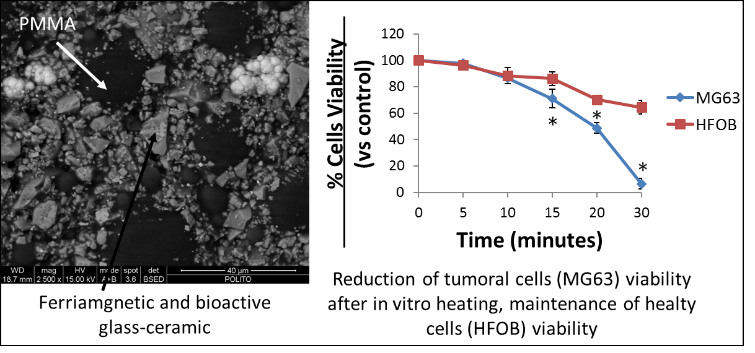
Bone cements based on polymethylmethacrylate (PMMA) are clinically used in several cemented prosthetic devices and to fill bone defects. Since '70 it has been suggested to introduce antibiotics directly into the PMMA-based cements used to fix prostheses to bone, in order to prevent bacterial proliferation at the interface between bone-cement and prosthesis. However, after releasing their antibiotics they act as foreign body to which bacteria preferentially adhere and grow, producing secondary infections and developing antibiotic-resistant strains. To solve this problem, the addition of a single inorganic phase contemporaneously antibacterial and bioactive (glasses doped with Ag+, Cu++… ) to the bone cement is suggested, joining, in this way, osteointegration and the antibacterial properties. Moreover, PMMA based bone cements are commonly used to fill large bone defects coming from surgical procedures on bone metastasis. In order to provide a better protection of the metastatic bone from the pathological fractures associated with tumors, composite bone cements containing a bioactive and ferrimagnetic glass-ceramics have been prepared. In this way the bone cement, usually used as inert bone filler, could induce bone ingrowth on its surface by osteoinduction mechanisms and, at the same time, could be used for non-invasive hyperthermic treatments of bone, by magnetic induction, selectively killing tumoral cells. Besides, the glass-ceramic can be doped with antibacterial element to reach antibacterial properties.
Picture(s)

Contact(s)
enrica.verne@polito.it
Recent reference(s)
-
Miola M. et al., Biomedical Materials, 2015, vol 10(5), 055014, doi:10.1088/ 1748-6041/10/5/055014
-
M. Miola et al., Mat. Sci. Eng. C. 43 (2014) 65-75, doi : 10.1016/j.msec.2014.06.026
-
Bruno M. et al., Journal of biomaterials applications, 29 (2014) 254-267. DOI: 0.1177/0885328214521847
-
E. Vernè et al., Materials Science and Engineering C 53 (2015) 95–103, doi: 10.1016/j.msec.2015.03.039
-
M. Miola et al., Ceramics International 43 (2017) 4831–4840, doi: 10.1016/j.ceramint.2016.12.049
-
Vernè E. et al., TO2009A000518, PCT/IB2010/053181, EP2451493A2.
Funding
- Regional Project on Applied Scientific research, 2003, Health and Medical Science sector: “Biomateriali per terapie non invasive nel trattamento in ipertermia dei tumori”.
- Regional project 2007-2009: Cementi per artroprotesi caricati con vetri bioattivi ad azione antibatterica